How, when, where; recommendations and interpretation
Air quality sampling and testing is a valuable practice to ensure food safety and stability; airborne contaminants can quickly and significantly reduce product quality and lead to health hazards for consumers. This is why environmental air sampling can be a valuable tool for ensuring the safety and quality of food during production.
Current Regulatory Requirements
The United States Department of Agriculture Food Safety Inspection Service (FSIS) and Food and Drug Administration (FDA) state that food should be produced in a manner that does not compromise public health, which may include addressing hazards associated with air quality. Currently, FSIS and FDA do not enforce ambient air sampling monitoring frequencies or define acceptable levels. Similar to the FSIS and FDA, Safe Quality Food (SQF) and the British Retail Consortium (BRC) require air sampling as part of their programs, but do not define acceptable levels. Therefore, better guidance is necessary for a food facility to make value-added decisions when implementing and evaluating air quality sampling.
Frequency of Air Sampling
The frequency of environmental air sampling in a food production facility depends on several factors, including the type of food product being produced, the size of the facility, and the potential sources of contamination. Currently, the FDA and SQF guidance support that air sampling should be performed at least once a year, with more frequent sampling recommended for facilities that produce high-risk products or have a history of contamination issues. With the current guidance, facilities face challenges when deciding on actions for a single annual sampling without acceptable levels defined.
A more appropriate and advantageous practice of air sampling would be to define a routine frequency that positions a facility to establish acceptable baselines. Baseline sampling may require several sampling points over a specified period to determine what a “normal” fluctuation in air quality may be for a specific facility. The value in routine sampling accounts for air quality fluctuations that may occur due to seasonality, production activities, or air filtration issues to name a few. For example, airborne yeast and molds may be more prevalent in summer and fall months when weather conditions are more favorable for outgrowth; this may also become evident when comparing indoor air sampling data. Sampling weekly, monthly, quarterly, or some other defined frequency provides a facility with the data to observe air quality fluctuation over time.
What to Sample For
Food production facilities can monitor contaminants in environmental air sampling, including microorganisms, such as bacteria and fungi; particulate matter, such as dust and allergens; and chemical contaminants, such as pesticides and volatile organic compounds (VOCs). The specific contaminants sampled depend on the type of food product produced and the potential sources of contamination.
Typically, air sampling in food production facilities focus on microorganisms; total plate counts (TPC), aerobic plate counts (APC), and total yeast & mold (Y&M) counts; all of which are appropriate non-specific assays to assess the quantity of ambient airborne microorganisms in an environment. These assays will support the growth of each single microorganism, known as a colony-forming unit (CFU), that will grow on the agar plate and later quantified. In certain scenarios, more specific assays may be used to quantify individual microorganisms or airborne particulates; for example, in a ready-to-eat (RTE) area, it may be appropriate to sample for Listeria monocytogenes during or after sanitation tasks since high-pressure cleaning activities can cause the pathogen to become aerosolized.
Collecting Ambient Air Samples
The most common and simplest air sampling method is passive sampling. Passive sampling involves the use of settling plates or contact plates that are placed in the air for a set period, allowing microorganisms to settle on to a nutrient rich agar plate. When implementing an air sampling program that uses passive sampling, it is critical to standardize the sampling time (i.e., 1 hour) so that the quantifiable colony forming units can be compared between samples.
Another option for air sampling is active sampling; active sampling uses a pump to draw air through a collection device and deposit the sample on an agar plate. Adjusting the air sampling pumps for sampling time and implementing standardized sampling time is essential for the air sampling procedure similar to passive sampling.
A third and less common option used in food production facilities is real-time monitoring, which utilizes electronic devices to detect and measure airborne particles, sometimes referenced as total suspended particulates (TSP), including microorganisms. This method allows for continuous monitoring of the facility and can provide immediate alerts to potential contamination events. Real-time monitoring may be implemented in laboratory settings where avoiding airborne contamination is more critical.
Establishing Air Sampling Baselines
Similar to other environmental sampling programs, establishing a baseline for environmental air quality in a food production facility is essential to identifying changes or deviations from “normal” conditions. Normal conditions and acceptable levels may differ across the food industry depending on the products being produced and sensitivity of the product to potential airborne contamination. For example, limiting or avoiding airborne contamination for RTE products may be more critical for reducing spoilage opportunities, extending shelf-life, and ensuring public health. Establishing a baseline for air quality involves sampling at multiple locations throughout the facility and at different times during production.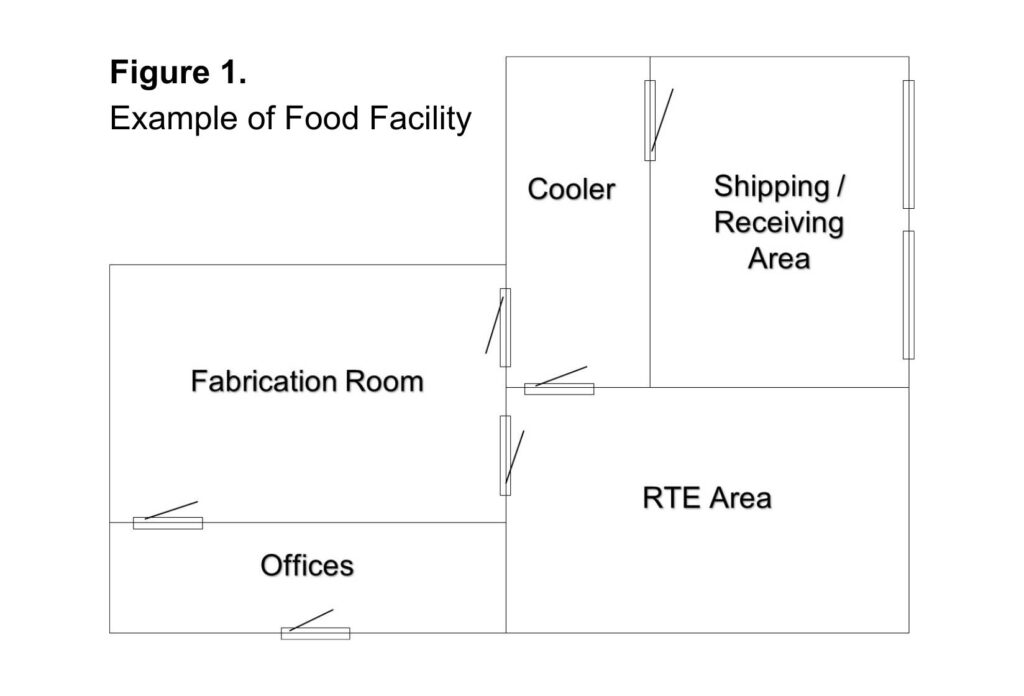
As a mock example, in a small production facility, a sampling program to establish baseline data may include sampling every month in numerous locations at different times. For instance, a sampling plan may include sampling prior to and during production in the fabrication room, RTE area, in the cooler, on the shipping/receiving dock, and in the office space. Fluctuations are readily observed and addressed within the year when monthly sampling regimes are replicated, by sampling at different times in different locations.
How to Interpret the Data
Once air samples are collected and the data is compiled, the results can be used to identify potential sources of contamination based on what is “normal” for the facility established from baseline data. For example, over a period of time, results may indicate that 120 CFU / plate from a 1-hour passive sampling is the acceptable operating range for the facility. In this case, any sampling point above that baseline may require a corrective action. Sampling points above the baseline may be due to seasonal changes (such as high levels of Y&M in summer months), as seen in figure 2. Another observation may show an adverse trend, such as figure 3, indicating that air filters need to be replaced or another change in the facility is compromising air quality. Facilities can implement adjustments and corrections to maintain air quality by evaluating the data compared to a baseline. (figure values do not necessarily establish acceptable ranges).
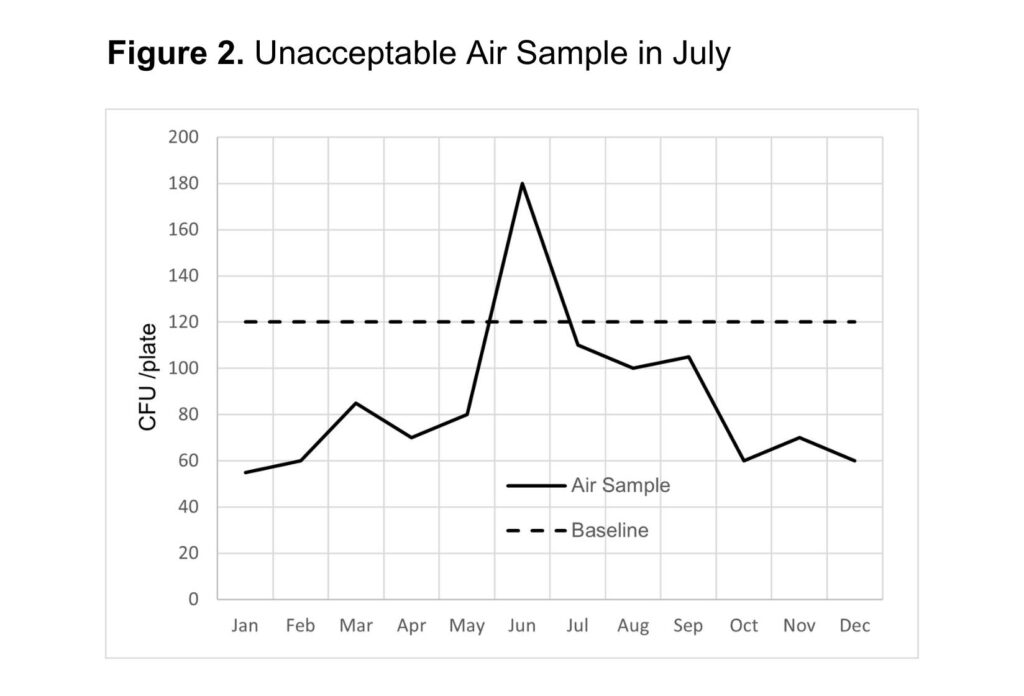
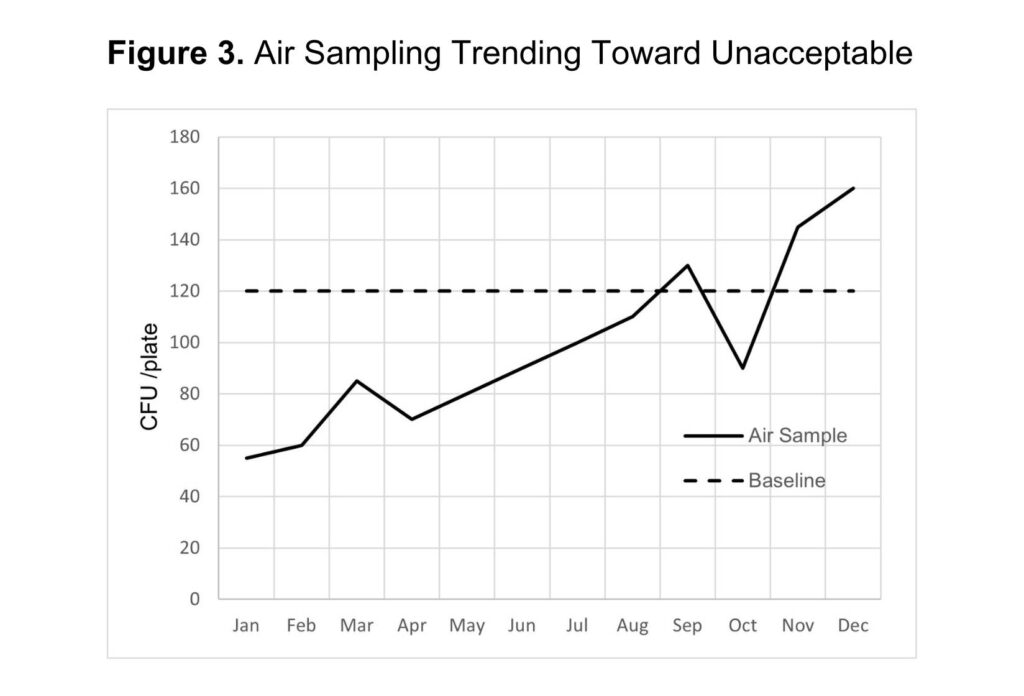
Air Quality Improvements
If high levels of airborne bacteria are detected, facilities should improve ventilation and reduce the potential for bacterial growth. Below are solutions to consider for continuously enhancing air quality.
- Implement or reevaluate cleaning and maintenance schedules: A clean environment is crucial in maintaining good air quality in a food facility. Regular cleaning of all surfaces, equipment, and ventilation systems can help prevent the buildup of contaminants and pollutants.
- Improve ventilation systems: Proper ventilation is essential for removing stale air, reducing humidity, and preventing the accumulation of airborne pollutants. Increasing the ventilation rate or introducing new air handling units can help improve air quality.
- Install air purification systems: Air purification systems such as air filters or UVGI (Ultraviolet Germicidal Irradiation) systems can help remove particulates, microorganisms, and other contaminants from the air.
- Control sources of pollution: Identifying and controlling sources of pollution within the facility, such as cooking or cleaning chemicals, can help prevent the release of harmful pollutants into the air.
- Educate employees: Educate on the importance of good air quality and the proper cleaning and maintenance practices needed to ensure the health and safety of the work environment.
Environmental air sampling is a valuable tool for ensuring the safety and quality of food production facilities. By establishing baselines and monitoring for potential contaminants, food producers can identify potential sources of contamination and take corrective actions to improve air quality and reduce the risk of contamination. The frequency and types of sampling will vary depending on the facility and the type of food product being produced. Still, regular monitoring is essential for maintaining safe and high-quality food products.
References:
21 CFR § 110 – Current Good Manufacturing Practice in Manufacturing, Packing, or Holding Human Food.
Food and Drug Administration. (2017). Control of Listeria monocytogenes in ready-to-eat foods: guidance for industry draft guidance. US Food and Drug Administration, Silver Spring, MD.
International Association for Food Protection. (IAFP, 2018). Environmental Monitoring for Control of Foodborne Pathogens: Best Practices, Challenges, and Innovations. Journal of Food Protection, 81(1), 71-83. doi: 10.4315/0362-028X.JFP-17-226.
SQF. (April 2021). Ambient Air Testing. Edition 9 Guidance Document.
USDA-FSIS. FSIS Directive 5000.1 Re. 7. Verifying an Establishment’s Food Safety System (10/20/2022).
Bedale, W. (2022). Environmental Controls: Emerging Technologies and Predictive Analytics to Address Complex Sanitation Challenges. Food Protection Trends, 42(4), 326-336.

Recent Comments